Legal Mentions and References
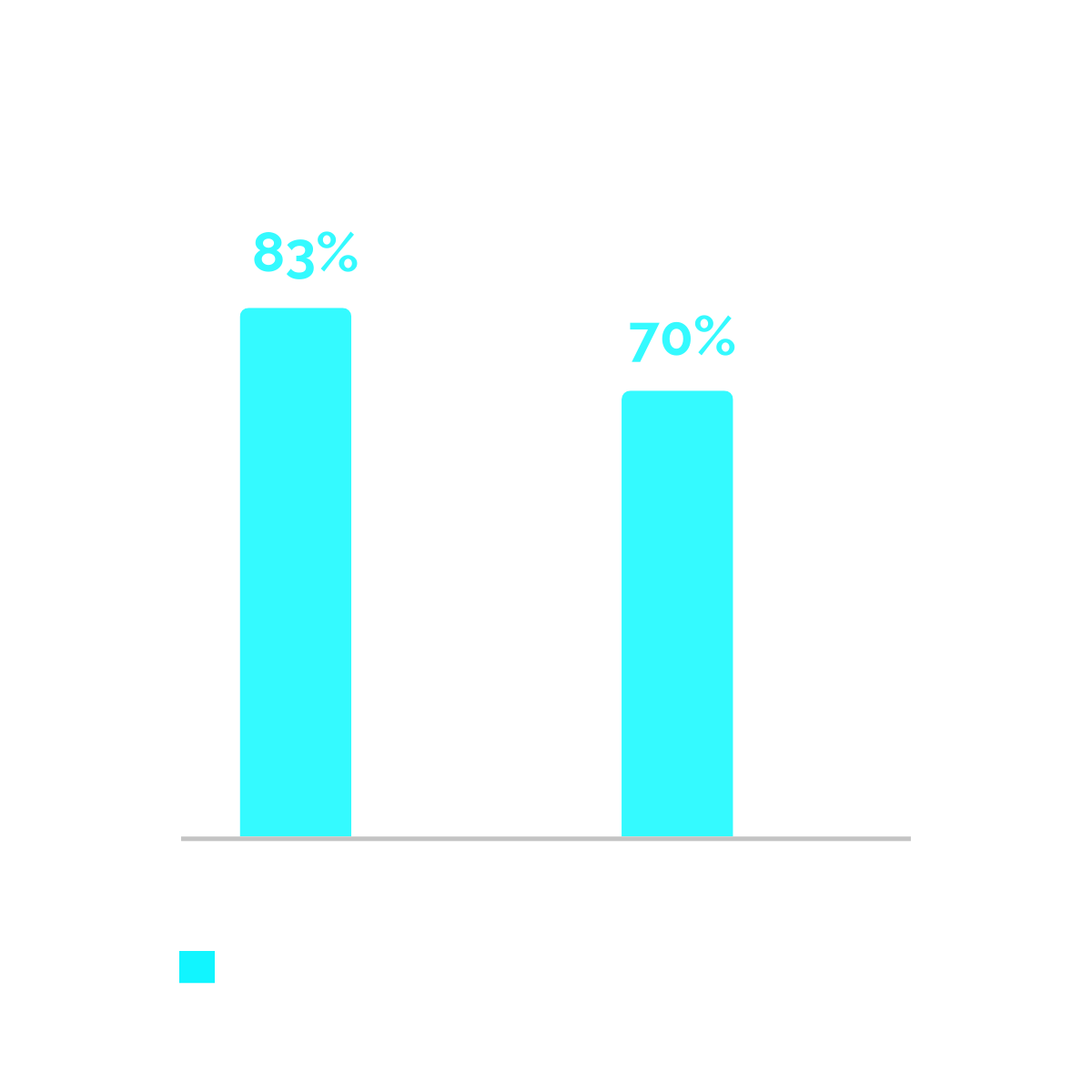
1 Distribution and Risk Profile of Paroxysmal, Persistent, and Permanent Atrial Fibrillation in Routine Clinical Practice” Chern-En Chiang, Lisa Naditch-Brûlé, Jan Murin, Marnix Goethals, Hiroshi Inoue, James O’Neill, et al.
2 Kirchhof et al.. "Catheter ablation in patients with persistent atrial fibrillation." European Heart Journal 38.1 (2017): 20-26.
ABOUT
The Volta AF-Xplorer™II system is a class IIa Medical Device manufactured by Volta Medical. This product is CE marked by TÜV Rheinland LGA Products GmbH (0197) and FDA cleared.
INDICATIONS, SAFETY & WARNINGS
CAUTION: Federal (United States) law restricts this device to sale by or on the order of a physician. See
Instructions for Use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events. All safety considerations, cautions and warnings that apply to the general use of a medical system in an operating room, also apply while using Volta AF-Xplorer™ II application. There are no known potential adverse events associated with the use of Volta AF-Xplorer™ II.
Indications: The Volta AF-Xplorer™ II assists operators in the real-time manual or automatic annotation of 3D anatomical and electrical maps of human atria for the presence of multipolar intra-cardiac atrial electrograms exhibiting spatiotemporal dispersion during atrial fibrillation or atrial tachycardia.
Contraindications: The Volta AF-Xplorer™ II has no specific contraindications.
All pictures shown are for illustration purpose only.
‡ Indicates a third party trademark, which is property of its respective owner.







.png)
 Access the full publication
Access the full publication
 Access the full publication
Access the full publication
.png)